Micromachining – electrochemical machining
1. Introduction to Electrochemical Machining. The chemical reactions involving the gain or loss of electrons that occur on the surface of the cathode and anode are known as electrochemical reactions. […]
1. Introduction to Electrochemical Machining.
The chemical reactions involving the gain or loss of electrons that occur on the surface of the cathode and anode are known as electrochemical reactions. The method of using these electrochemical reactions for machining is called electrochemical machining (ECM). Based on the principles of electrochemical machining, the technology can be divided into two categories: subtractive manufacturing based on the principle of anodic dissolution, including electrochemical machining, electrochemical polishing, and electrochemical deburring; and additive manufacturing based on the principle of cathodic deposition, including electroforming, electroplating, and electro-brush plating. Compared to electrical discharge machining (EDM), electrochemical machining offers advantages such as higher material removal rates, no heat-affected zone, smooth surface finish, and no tool wear.
In a chemical reaction, when a metal comes into contact with its salt solution, electron exchange often occurs, where the metal donates electrons to the ions in the solution and gains electrons from the solution. When this electron exchange reaches equilibrium, a thin double layer forms on the metal surface. The surface of a chemically active metal carries a negative charge, while the solution carries a positive charge, and the surface of a less reactive metal carries a positive charge while the solution carries a negative charge. The presence of the double layer creates a potential difference between the metal and its salt solution. This potential difference, which is generated due to the equilibrium between the dissolution and deposition of the metal in the salt solution, is called the equilibrium electrode potential. When a metal is immersed in another electrolyte, it also forms a double layer and potential difference. If two metal electrodes are connected by a conductor, electrons will flow through the conductor, creating a primary cell. Electrons flow from the iron electrode to the copper electrode, and this electron flow is extremely slow. Electrochemical machining technology utilizes this principle of electron flow.
Our factory business: carbide parts, mold parts, medical injection molds, precision injection molds, teflon PFA injection molding, PFA tube fittings. email: [email protected],whatsapp:+8613302615729.
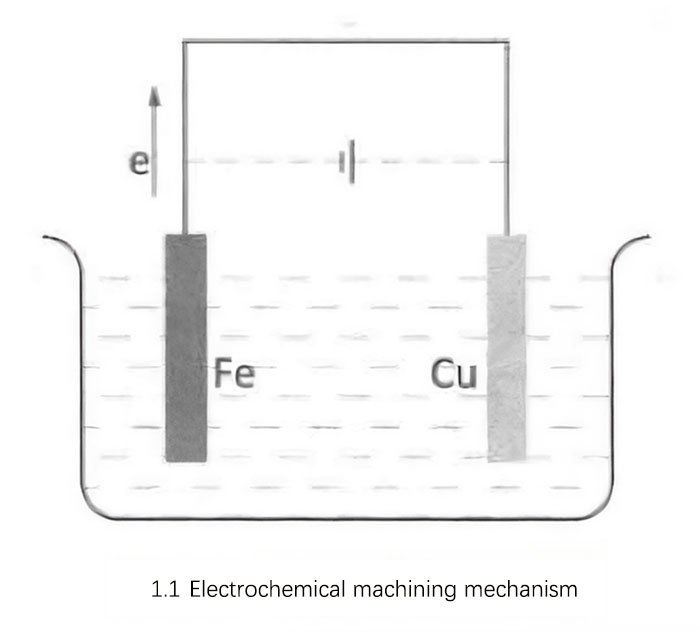
As shown in Figure 1.1, during the machining process, an external electric field is applied to accelerate the flow rate of electrons, thus increasing the rate of chemical reactions and achieving the removal of metal material. This method of applying an external electric field is equivalent to combining an electrolytic cell with the primary cell. After applying power, under the influence of the electric field force, the cations in the electrolyte move towards the cathode (Cu electrode), and the anions move towards the anode (Fe electrode). The external power source continuously draws electrons from the anode, causing rapid dissolution of the anode metal ions, while simultaneously supplying electrons to the cathode, resulting in the deposition of cations.
2. Characteristics of Electrochemical Machining Technology.
- It can process difficult-to-machine metal materials with high strength and hardness, such as tungsten carbide, titanium carbide, and high-temperature alloys. Moreover, the machining rate is not dependent on the mechanical properties of the metal. It can be used to process complex cavities on the surface of high-strength materials, such as aircraft engine blades, rocket engine nozzles, integral impellers, and various complex two-dimensional or three-dimensional holes and surfaces.
- During the machining process, there is no generation of cutting forces or cutting heat, making it particularly suitable for processing thin-walled parts that are prone to deformation. Electrochemical machining is a cold machining method performed in the form of ions. There are no residual stresses or heat-affected zones in the machining process, resulting in good surface quality of the workpiece without burrs or flash.
- The tools used in the machining process have no wear and can be used for a long time. However, it is necessary to prevent the deposition of cathodic products and the effects of short-circuit burns on the cathode of the tool. Electrochemical machining has high efficiency, especially in electrochemical machining, where the material removal rate is much higher than that of electrical discharge machining.
- Electrochemical machining technology can only process conductive materials. Compared to traditional mechanical machining, electrochemical machining equipment requires larger investment and occupies more space. Additionally, the electrolyte used may corrode the equipment, and the electrolytic products may have environmental impacts.






